Methanation
Methanation system
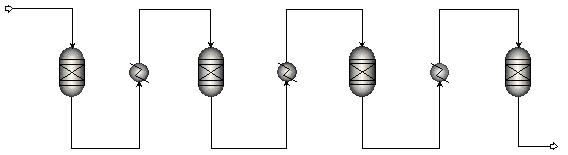
Due to the high exothermic character of the methanation reactions the temperature will increase significantly in adiabatic systems. Resultantly, the thermodynamic equilibrium is readily reached but with only limited conversion. To achieve high conversions the temperature must be decreased, i.e. the reaction heat has to be removed. Typically, this is achieved by internally cooled reactors or by gas recycles as in the commercial processes of e.g. Haldør-Topsoe and Lurgi. The simplest system, however, comprises a series of (adiabatic) methanation reactors with intermediate heat exchangers (figure below). The application of such a system is limited to processes at lower pressures as at higher pressures the adiabatic temperature increase in the reactors will result in too high temperatures and thermal damage of the catalysts.
In each adiabatic reactor the methanation reaction will take place till thermodynamic equilibrium is reached. Two effects determine the methane equilibrium concentration:
1. Formation of CH4 due to the reaction of H2 and CO;
2. Conversion of CH4 due to the increase in temperature (steam reforming of methane).
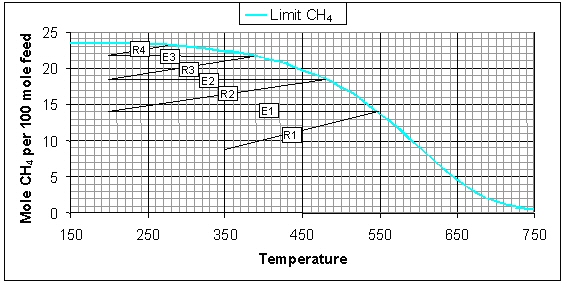
In the figure below the variation of the CH4 concentration is illustrated for the four methanation reactors as well as the adiabatic temperature effects. Each diagonal line represents an adiabatic reactor and each horizontal line represents intermediate cooling. The initial CH4 flow is 8.9 mol/h (total flow of wet feed gas is 100 mol/h) and the inlet temperature of the first methanation reactor R1 is 350°C. In the reactor the amount of CH4 increases to 13.7 mol/h, which is accompanied by an adiabatic temperature increase of 199°C (outlet temperature is 549°C). The first cooler E1 cools the gas to 200°C, i.e. the inlet temperatures of the reactors R2, R3, and R4. After the fourth reactor E4 the CH4 flow is 23.1 mol/h, which corresponds to a CO conversion of 98.3%.
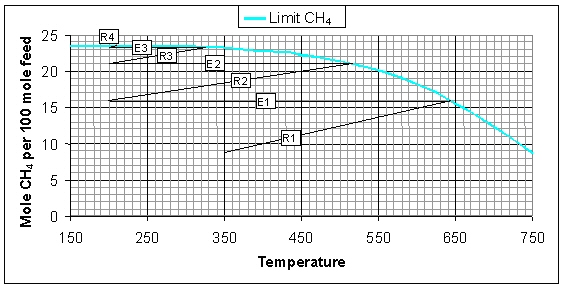
At higher system pressures the thermodynamic equilibrium will shift towards higher conversion and more CH4 formation, resultantly, the adiabatic temperature increase will be higher. In the figure below the variation of the CH4 flow and temperature in the adiabatic systems at 10 bar is illustrated. The conversion at 10 bar is higher compared to the system at 1 bar, i.e. the conversion after the third reactor R3 is even higher than after the fourth reactor R4 in the 1 bar system. Furthermore, the higher pressure has an advantageous effect on the catalyst activity due to which less catalyst is required to reach the same conversion. The amount of CH4 that is formed is 23.5 mol/h, which corresponds to almost 100% conversion.
Experimental
In an experimental programme various commercial available methanation catalysts were evaluated on their suitability for application in the bio SNG system with adiabatic reactors. The evaluation as included in the project was limited to the determination of the initial activity of the catalysts.
The experiments were performed in a fully automated ‘parallel flow’ set-up with six 4 mm diameter quartz reactors to allow testing of six samples under identical conditions, i.e. pressure, temperature, and feed gas composition. Maximum pressure is 5 bar and maximum temperature is 550°C. Isothermal operation is approached by the use of thin reactors and nitrogen as dilution gas; the lower partial pressures of the reactants are compensated by applying a higher pressure (i.e. 3 bar). A gas chromatograph analyses the reactor off-gases every four minutes.
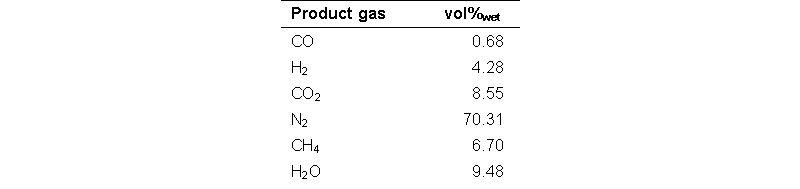
A typical test run comprises a step-wise increase of the temperature and determined the temperature related rate of formation of methane per gram of catalyst (i.e. the activity). Composition of the feed gas for the catalyst screening tests is shown in the table below.
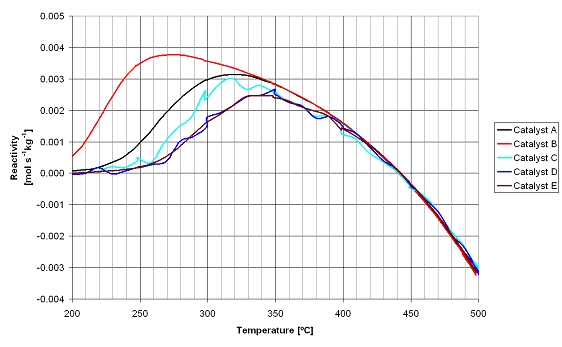
In the figure below the results of the activity experiments are shown. The curves for catalysts C to F are not smooth, which is the result of the gas analysis method and not related to the catalyst performance. Catalyst B has the highest activity, i.e. it exhibits already activity at 200°C and at 250°C the activity is already three times higher than catalyst A. The order of activity for the tested catalysts is B >> A > C > D E. The negative methane formation rate observed at temperatures above 440°C is due to the methane reforming reaction that is taking place, as at higher temperatures the thermodynamic equilibrium concentration of methane is lower than the feed gas concentrations. Catalyst B was selected for the application in the methanation section in the integrated line-up for the demonstration of the technical feasibility.