Indirect gasification results
The lab-scale MILENA has been operated during a large number of tests under different conditions. Parameters that have been varied are biomass fuel, gasification temperature, bed material, inertisation gas and supplementary fuel to combustor (simulating tar recycle). The aim of the experiments was find the optimum conditions for the high-efficient production of a CH4-rich product gas.
Biomass fuel
Two different types of fuels were used in the tests: clean beech wood and grass. Beech wood was fed as small particles (source: Rettenmaier Benelux, “Rauchergold”) and grass was fed as milled pellets (source: “Ekogras” form Hartog Grasdrogerij B.V). The fuel compositions are given in the table below.
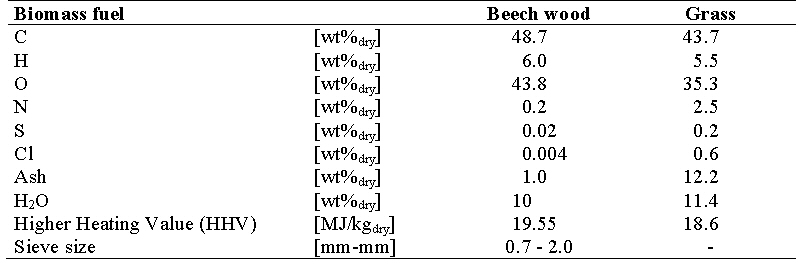
Grass was selected to investigate the sensitive of the MILENA towards agglomeration of the bed material. Agglomeration of the bed material can happen when a sticky layer on the bed particles is formed and one factor that enhances the formation of this layer is the presence potassium. Grass contains a relatively large quantity of potassium compared to wood.
In experiments with wood, agglomeration was never observed. In the experiment with grass, agglomeration occurred within twenty minutes resulting in a shutdown of the gasifier (temperatures in the riser and combustor were 810 and 882°C, respectively). A possible solution to prevent agglomeration is the lowering of the process temperature. However, this was not further investigated within the scope of this project. All further experiments were carried out with beech wood as fuel.
Gasification temperature
The gasification temperature influences the product gas composition, the amount and composition of the tar in the gas, and the conversion of the fuel in the gasifier. The gasifier temperature is measured at the outlet of the gasifier. A thermocouple placed in the gas stream is used for this measurement. The heat loss in the upper part of the installation is relatively high. This causes a rapid decrease in gas temperature at the outlet. In previous experiments, the temperature was measured in the settling chamber, were there was a direct contact between thermocouple and circulating sand, this temperature measurements gives an better indication of the gasifier temperature, but the thermocouple broke down. The average difference in measured gas temperature was 26.5°C. The gasification temperature, reported here, is defined as the measured gasifier outlet temperature +26.5°C.
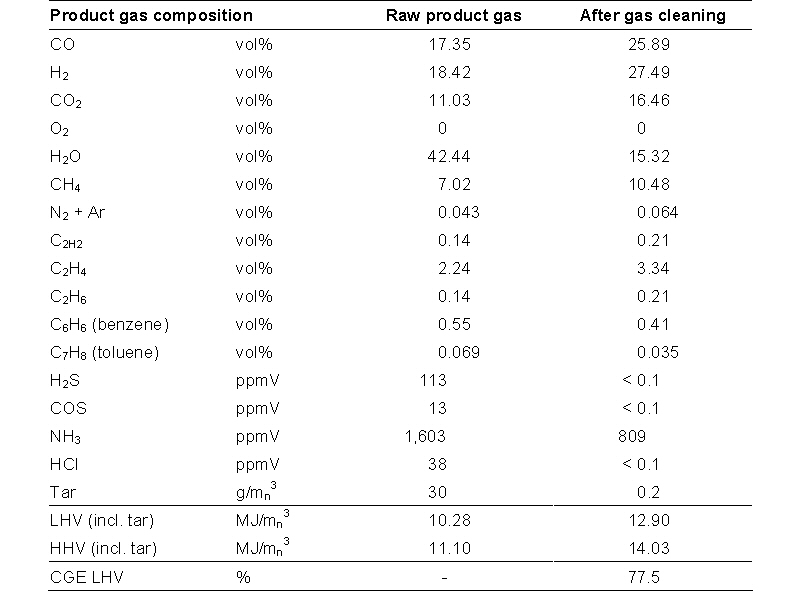
By varying the reactor wall temperature (trace-heating) and adding additional fuel to the combustor (both the use of recycled product gas and recycled tar were simulated by oil for practical considerations) the temperature in the reactor was varied. The air to fuel ratio for the combustor was held at a fixed value (typical between 3 and 6 vol% dry of oxygen in the flue gas). In a commercial installation (and the foreseen MILENA pilot plant) the gasifier temperature is not a control parameter but a result of the temperature in the combustor, which is set by the amount of char that is fed to the combustor. The table below gives the gas composition for the lab-scale MILENA gasifier as a function of temperature. As can be seen from the data, the concentration of methane typically decreases with increasing the temperature.
Effect on tar formation
The total amount of tar produced in the gasifier without the use of catalytic bed material is relatively high and varies a lot. Increasing the temperature does not decrease the total amount of tars in the gas (table 3.2). Heterocyclic components, like phenol, pyridine and cresol (class 2 tars) decrease in concentration with increasing temperature. Heavy poly-aromatic hydrocarbons (4-5 rings PAH’s, i.e. class 5 tars) increase in concentration with increasing temperature. The heterocyclic tar components are the least stable and therefore readily broken down. The heavy poly-aromatic hydrocarbons are formed from lighter tars (i.e. via polymerization). This behaviour is also observed in bubbling fluidized and circulating fluidized gasifier [1].
Effect on fuel conversion
The fuel conversion (or carbon conversion) in the gasification section of the installation varies between 70 and 90%. The unconverted fuel (char) is send to the combustor were it is completely combusted and produces the heat for the gasification reactor. Resultantly, the fuel conversion in an indirect gasifier system is essentially 100%. The amount of char going to the combustor determines the temperature in the gasifier, so the fuel conversion in the gasification reactor determines the temperature in the combustor and the gasifier. This makes the carbon conversion in the gasification section an important design parameter.
Carbon conversion is defined as the amount of carbon in the product gas divided by the amount of carbon in the fuel or 100% minus the amount of solid carbon leaving the gasifiers divided by the amount of carbon in the fuel. The last method was used to calculate the carbon conversion in this report. The amount of solid carbon leaving the gasifier was calculated from the amount of air fed to the combustor and the measured oxygen concentration in the flue gas. Part of the carbon leaves the system with the product gas in the form of dust and is not returned to the combustor in the lab-scale installation. The product gas contains approximately 10 g/mn3 of dust. An estimated 20 wt% of this dust is bed material (sand). Normally 5 mn3/h of gas is produced; this results in a char loss of 40 gram/h. Corresponding to approximately 10% of the char that is produced in the gasifier.
Carbon conversion is influenced by fuel particle size, fuel type, temperature, and residence time in the gasifier. The particle size cannot be varied in a range that is useful for commercial application, because the size of the feeding system and the reactor is relatively small in the lab-scale set-up. For all test fuel particles of 0.7 2.5 mm were used. For commercial applications particles up to several cm are foreseen. Tests in a pilot-scale plant must generate the required carbon conversion fuel size relations.
The calculated carbon conversions, defined as the percentage of the carbon in the fuel converted to carbon in the product gas (CO, CO2, CH4, CxHy, tar), for the different temperatures are given in table 3.2. The carbon conversion generally increases with increasing temperature. This makes the process self-regulating, if the temperature in the reactor lowers, the amount of char produced increases and the amount of heat produced in the combustor increases. Resulting in an increase in gasification temperature.
Recycle of tar to the combustor
Tar recycle was simulated by the supply of oil to the combustor, because it was not possible to feed relatively small quantities of tar in the lab-scale set-up. A cooled nozzle was fabricated to feed the oil in the bed (near the bed wall). The temperature in the bed was increased by the combustion of the oil. The increased combustion reactor temperature resulted in an increased gasifier temperature.
The table below gives the gas composition of the flue gas leaving the combustor. Secondary air (approximately 10%) was injected in the freeboard during all tests to improve the combustion. The reference composition given in the first column is an average of all tests done without injection of oils or methane.
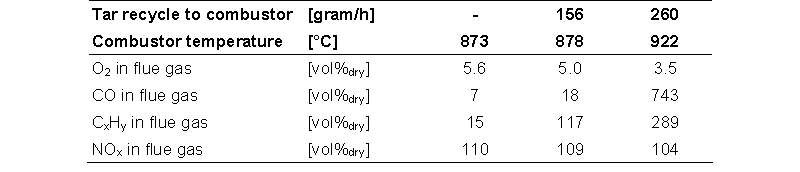
The tar / oil recycle had a negative impact on the flue gas emissions of the combustor. The concentrations of CO and CxHy increased. The local injection of the oil is probably the reason for the incomplete combustion. Without an oil / tar recycle to the combustor the emission of CO and CxHy are below the emission limits for waste streams in the Netherlands. For the injection of tars to the gasifier a properly designed injection nozzle system is required, otherwise the emissions of CO and CxHy will increase to an unacceptable level.
Alternative bed materials
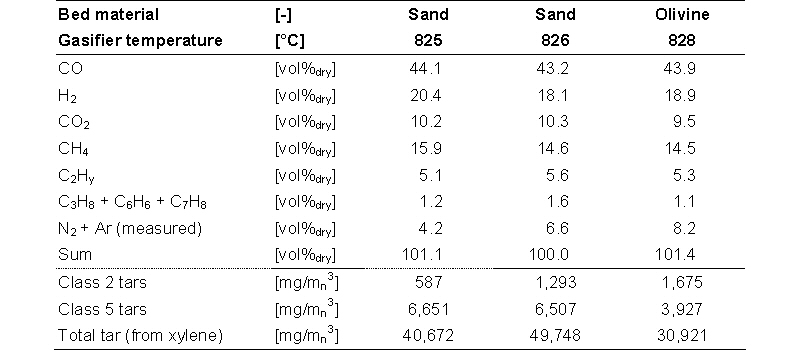
Sand is used as the standard bed material, because it is cheap and resistant to abrasion. Other fluidized bed gasifiers use also olivine and dolomite as bed material, because these materials are catalytic active for the reduction of tar in the product gas. Dolomite is relatively soft and has a high abrasion rate in typical fluidized bed applications. Furthermore, fresh dolomite calcines (i.e. reaction of CaCO3 to CaO and CO2) in the reactor, which is an endothermic reaction that decreases the gasifier efficiency. Olivine is much harder then dolomite and seems a good alternative to sand. The FICFB gasifier in Güssing (Austria) used olivine in a bubbling fluidized bed as bed material
As can be seen from the gas composition in the table below the decrease of the tar content by the use of olivine instead of sand is not significant. A good contact between the catalyst and the product gas is required for an optimal tar reduction. In the MILENA reactor the biomass is gasified in a riser. Compared to a fluidized bed the contact between the produced gas and the bed material (catalyst) is less optimal. The use of catalytic bed material in the MILENA does not yield advantages.
Nitrogen dilution in product gas
The product gas from an indirect gasifier contains small amounts of nitrogen. The nitrogen comes from air that is fed with the fuel, nitrogen that is used as purge gas, fuel-bound nitrogen, and gas transport from the combustor. Nitrogen in the product gas increases in N2 concentration in the final SNG product. Experiments were performed to minimise the nitrogen dilution resulting from the use of nitrogen as inertisation gas of the biomass feeding bunkers. The fuel bunker was purged with CO2 and the nitrogen purge of the feeding screw was replaced by a CO2 purge. A CO2 purge is a realistic option for commercial plant, as CO2 is removed in the SNG upgrading, therefore CO2 is available and a CO2 dilution of the product gas is not a problem.
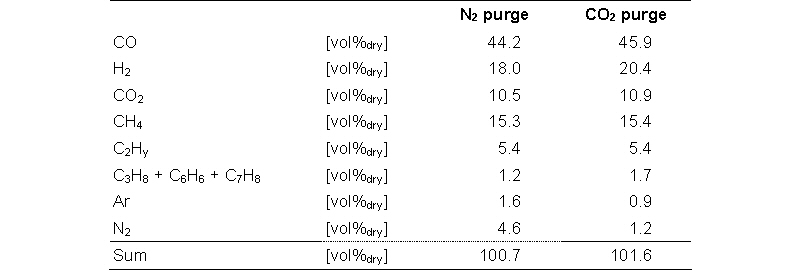
The compositions for a typical product gas produced in the lab-scale MILENA gasifier with and without a CO2 purge are shown in the table above. The argon in the gas results from the steam generator; argon is used as carrier gas. The nitrogen content can be as low as 1.2 vol% in the dry product gas, which results in a calculated N2 content in the SNG of approximately 2.5 vol%.
References:
S.V.B. van Paasen and J.H.A. Kiel: Tar formation in a fluidised-bed gasifier: Impact of fuel properties and operating conditions. ECN, ECN-C--04-056, 2004, The Netherlands.